

新闻动态
News Center
Big Event | China's 25 international business standards for plant extracts have been completed
- Categories:Industry information
- Author:
- Origin:
- Time of issue:2020-11-05
- Views:0
(Summary description)Recently, the official website of the China Chamber of Commerce for Import and Export of Medicines and Health Products (hereinafter referred to as the Chamber of Commerce for Medical Insurance) announced the third batch of 12 international business standards for plant extracts, namely white willow bark extract, betel nut polysaccharide polyphenols, and seaweed extract (Fucoida). Xanthin), Polygonum Resveratrol, Honeysuckle Extract (5% Chlorogenic Acid), Honeysuckle Extract (25% Chlorogenic Acid), Ganoderma Extract (Water Extract), Monk Fruit Extract (25% Mogroside V) , Monk fruit extract (50% Mogroside V), green coffee bean extract, chlorogenic acid, stevia extract. So far, the Medical Insurance Chamber of Commerce has completed the formulation of 25 international business standards for plant extracts.
Big Event | China's 25 international business standards for plant extracts have been completed
(Summary description)Recently, the official website of the China Chamber of Commerce for Import and Export of Medicines and Health Products (hereinafter referred to as the Chamber of Commerce for Medical Insurance) announced the third batch of 12 international business standards for plant extracts, namely white willow bark extract, betel nut polysaccharide polyphenols, and seaweed extract (Fucoida). Xanthin), Polygonum Resveratrol, Honeysuckle Extract (5% Chlorogenic Acid), Honeysuckle Extract (25% Chlorogenic Acid), Ganoderma Extract (Water Extract), Monk Fruit Extract (25% Mogroside V) , Monk fruit extract (50% Mogroside V), green coffee bean extract, chlorogenic acid, stevia extract. So far, the Medical Insurance Chamber of Commerce has completed the formulation of 25 international business standards for plant extracts.
- Categories:Industry information
- Author:
- Origin:
- Time of issue:2020-11-05
- Views:0
Recently, the official website of the China Chamber of Commerce for Import and Export of Medicines and Health Products (hereinafter referred to as the Chamber of Commerce for Medical Insurance) announced the third batch of 12 international business standards for plant extracts, namely white willow bark extract, betel nut polysaccharide polyphenols, and seaweed extract (Fucoida). Xanthin), Polygonum Resveratrol, Honeysuckle Extract (5% Chlorogenic Acid), Honeysuckle Extract (25% Chlorogenic Acid), Ganoderma Extract (Water Extract), Monk Fruit Extract (25% Mogroside V) , Monk fruit extract (50% Mogroside V), green coffee bean extract, chlorogenic acid, stevia extract. So far, the Medical Insurance Chamber of Commerce has completed the formulation of 25 international business standards for plant extracts.
New U.S. bill tightens supervision over plant extract imports
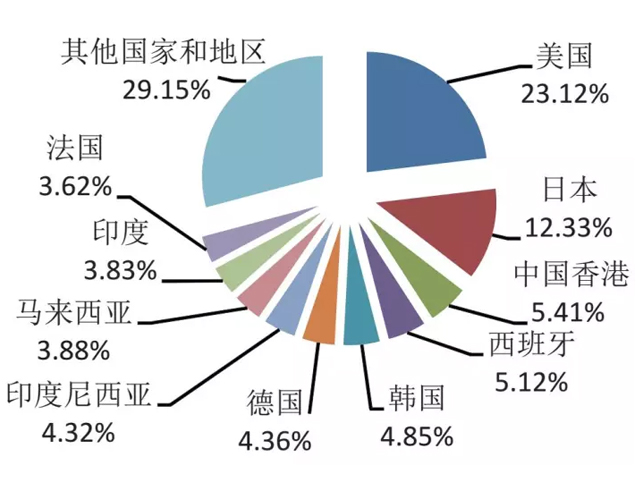
Figure 1 Top 10 export markets of plant extracts in my country from January to August 2017
Plant extracts are widely used in food, medicine, chemical industry, etc., and are the main force in my country's export of traditional Chinese medicine products. In recent years, with the stricter food and drug safety supervision in the international market, my country's plant extract products have ended the rapid growth of exports for many years and entered a period of adjustment. The whole industry urgently needs to improve the quality level and establish and improve industry standards.
Figure 2 The export trend of my country's top ten export markets of plant extracts from January to August 2017
According to the data of the Medical Insurance Chamber of Commerce, from January to August this year, my country's export value of plant extracts reached 1.31 billion US dollars, accounting for more than 60% of my country's total exports of traditional Chinese medicine products, but the export value fell by 3.14% year-on-year. Weak.
my country's plant extract exports are mainly concentrated in the three major markets of Asia, North America and Europe. Asia is a traditional market for my country's plant extract exports. From January to August this year, the export value of my country's plant extracts to the Asian market was US$580 million, a year-on-year decrease of 6.45%.
However, exports to Japan and my country's Hong Kong region have changed from last year's decline, and the export value has increased by 10.73% and 20.97% year-on-year respectively; the decline in the export of essential oil products to the ASEAN market has narrowed. From January to August, my country's export of plant extracts to the European market was relatively stable, with an export value of 290 million US dollars, a year-on-year increase of 1.7%; exports to North America were 320 million US dollars, a year-on-year increase of 7.31%, of which exports to the United States reached 300 million. U.S. dollars are mainly exported as dietary supplements and food raw materials (see Figure 1 and Figure 2 for the top ten markets and trends in my country's plant extract exports).
It is worth noting that the United States and other countries have become more and more strict with the import of plant extracts. On May 30 this year, the "Final Regulations for Foreign Supplier Verification System" (FSVP) issued by the United States was fully implemented, and the United States has tightened the audit and related supervision of domestic importers and foreign suppliers.
The above regulations require that the foreign supplier must be an enterprise that actually produces food and must have a corresponding U.S. consignor or consignee. If the food is imported without a U.S. consignee or consignee, the foreign owner of the food must designate a A U.S. agent who is responsible for ensuring that supplier verification activities are performed for each food item imported.
Verification activities are primarily annual on-site audits, sampling and testing of suppliers' facilities, or review of relevant documents such as a review of suppliers' food safety records. If the U.S. importer fails to perform FSVP verification, the U.S. Food and Drug Administration (FDA) will require the foreign supplier’s product to be returned or destroyed.
Therefore, relevant experts of the Medical Insurance Chamber of Commerce remind Chinese plant extract companies that they must carefully select buyers to ensure that they have undergone FSVP verification, and they need to prepare for their extended verification. The FSVP regulations have a greater impact on Chinese plant extract traders. Since they are not manufacturers, extended verification will become difficult.
Business standards help the internationalization of the plant extraction industry
Under the general environment of improving food and drug quality supervision standards in the international market, my country's plant extract industry standards are not perfect, and the compatibility with international standards is not strong, which makes exports easily affected. It is understood that there are more than 1,000 varieties of plant extracts in my country, 47 varieties are included in the Chinese Pharmacopoeia, 60 national food additive standards, 5 standards involving plant extracts formulated by the Ministry of Commerce, and the Ministry of Forestry and the Ministry of Agriculture. There are 5 standards related to extracts. Obviously, the existing standards are difficult to meet the development needs of the plant extract industry.
Facing the dual pressure of strengthening international supervision and weak exports, since 2012, the Medical Insurance Chamber of Commerce has organized domestic excellent plant extract companies to jointly carry out the formulation of the "International Business Standards for Plant Extracts" (hereinafter referred to as "Business Standards"). At present, business standards have become one of the considerations for relevant international customers to measure product quality, and even become a reference for inspection and quarantine at some ports. It is reported that the Medical Insurance Chamber of Commerce, through cooperation with the China National Institute for Food and Drug Control, plans to form a special set of plant extract standards for business standards, which may become an important source of health food filing raw material catalogs in the future. The Medical Insurance Chamber of Commerce also actively promotes the international mutual recognition of business standards. In cooperation with the US Pharmacopoeia, it is expected to form a set of mutual recognition standards between China and the United States in the future. It will make every effort to promote the entry of business standards into the US Pharmacopoeia, promote the development of Sino-US trade, and help Chinese enterprises to master The right to speak in foreign trade negotiations.
According to the relevant person of the Medical Insurance Chamber of Commerce, the organization of the international business standards for plant extracts is in accordance with GB/20004.1-2016 "Group Standardization Part 1: Guidelines for Good Behavior" and GB/T20003.1-2014 "Special Procedures for Standard Formulation No. 1" Part: According to the requirements of "Standards Involving Patents", the selection of varieties closely follows the market demand. The form and content of the standards are based on the "Pharmacopoeia of the People's Republic of China (2015 Edition)" and "USP39-NF34" and other authoritative standards at home and abroad. Grasp the development of domestic and foreign drug quality control. The review work invited relevant personnel from China National Institute for Food and Drug Control, domestic core scientific research institutes, and the standard collection department of the United States Pharmacopoeia, forming a strong team of experts.
Through the joint efforts of all parties, a total of 25 plant extract standards in three batches have been published, and these standards fill the blank of my country's plant extract standards. Yu Zhibin, director of the Chinese Medicine Department of the Medical Insurance Chamber of Commerce, said that the standards are dynamic and need to be constantly revised and updated with changes in the industry. At present, the fourth batch of commercial standards for plant extracts is being formulated and is expected to be announced in June next year. "Our goal is to make the standard cover all plant extract products, and we hope that more industry colleagues can join the medical insurance chamber of commerce to participate in the standard construction and jointly promote the internationalization of my country's extract industry." Yu Zhibin said.
Scan the QR code to read on your phone
江苏天晟药业股份有限公司
©2022 JIANGSU TIANSHENG PHARMACEUTICAL CO.,LTD. 苏ICP备14003387号

